The FDA Bait-and-Switch on Pfizer Covid-19 Jab
By The Sharp Edge
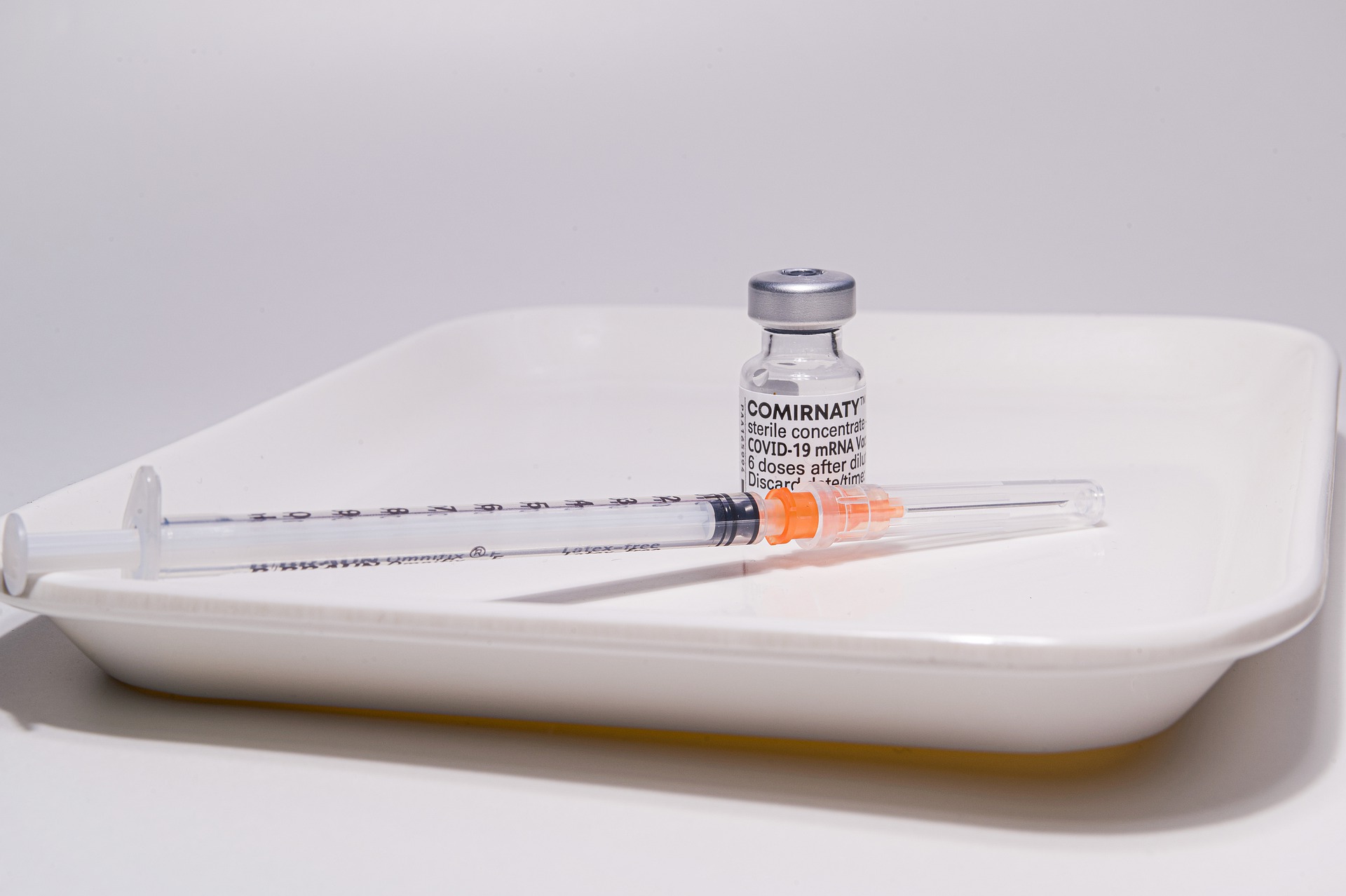
On August 23, 2021, the FDA announced their approval of the first Covid-19 jab. “The vaccine has been known as the Pfizer-BioNTech COVID-19 Vaccine, and will now be marketed as Comirnaty (koe-mir’-na-tee), for the prevention of COVID-19 disease in individuals 16 years of age and older. The vaccine also continues to be available under emergency use authorization (EUA), including for individuals 12 through 15 years of age and for the administration of a third dose in certain immunocompromised individuals,” the news release stated.
The legacy media quickly picked up their talking points and ran with them. Soon after, mandates swept across the public, private and academic sectors. “Let me say this loudly and clearly,” Biden announced, “If you’re one of the millions of Americans who said that they will not get the shot…until it has full and final approval of the FDA, it has now happened. The moment you’ve been waiting for is here. It’s time for you to go get your vaccination and get it today.” Understandably so, the public has been led to believe that the jab currently on the market has full FDA approval – which is nothing short of a bait-and-switch.
While the FDA did offer BLA approval for BioNTech’s Covid-19 jab labeled “COMIRNATY,” (which is currently in limited supply in the United States) the Food and Drug Administration also provided an extension of the EUA for the Pfizer BioNTech Covid-19 jab (of whichRead More